Conservatory Lab students have kept learning about the real world with hands-on activities even when we were all dependent on screens for communication with their teachers and peers. Thanks to a grant from the Cedar Tree Foundation, Conservatory lab launched the brand new Amplify science curriculum this fall. Students in Grade 5 have been using it to explore chemistry with digital simulations and hands-on experiments through an everyday question:

“How do you make the perfect salad dressing?”
As students arrived to their virtual classroom for their hour-long synchronous learning session in December, Science teacher Linden Mckenzie started class by putting up slides, setting expectations, and inviting students to find their materials in the learning kits, labeled 3-1.
Once students had their materials, Mx. Mckenzie captured their attention with a warm-up demonstration. As they prepared the materials, students were instructed to write a hypothesis in their virtual log about how water would mix with oil by using evidence from past experiments to support their theory. Students were then asked to unmute and share some ideas about what might happen.
One student wondered if the oil would create an exciting effervescent reaction similar to the vinegar and baking soda experiment they did last week. Another student thought the oil would mix with the water changing the color, and a third student thought the oil and water would not stick to each other.
To vote on which hypothesis was most likely, Mx. Mckenzie invited students to participate the way they would in a classroom setting with a silent visual vote raising one finger for hypothesis A, two fingers for B, and three fingers for C.
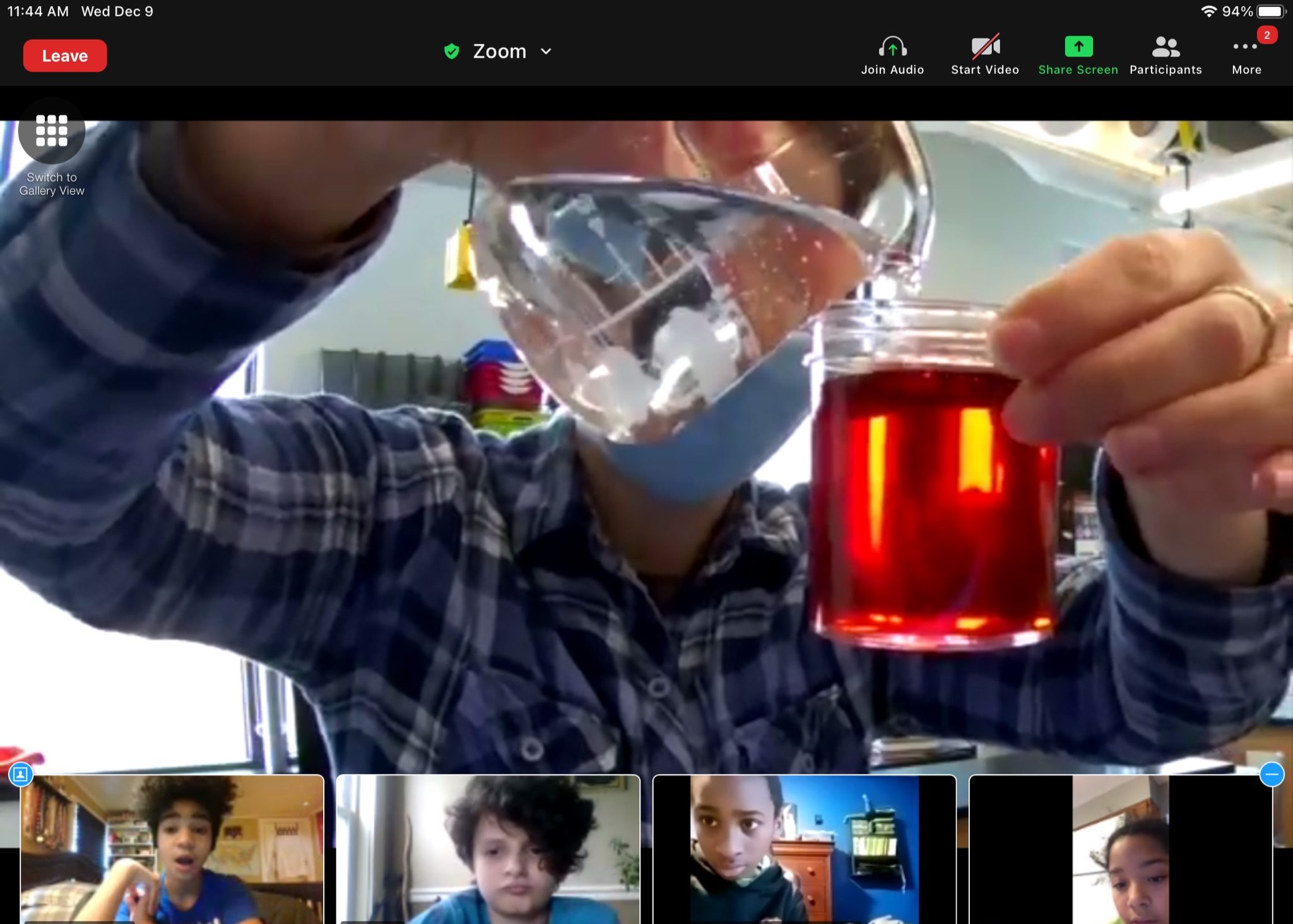
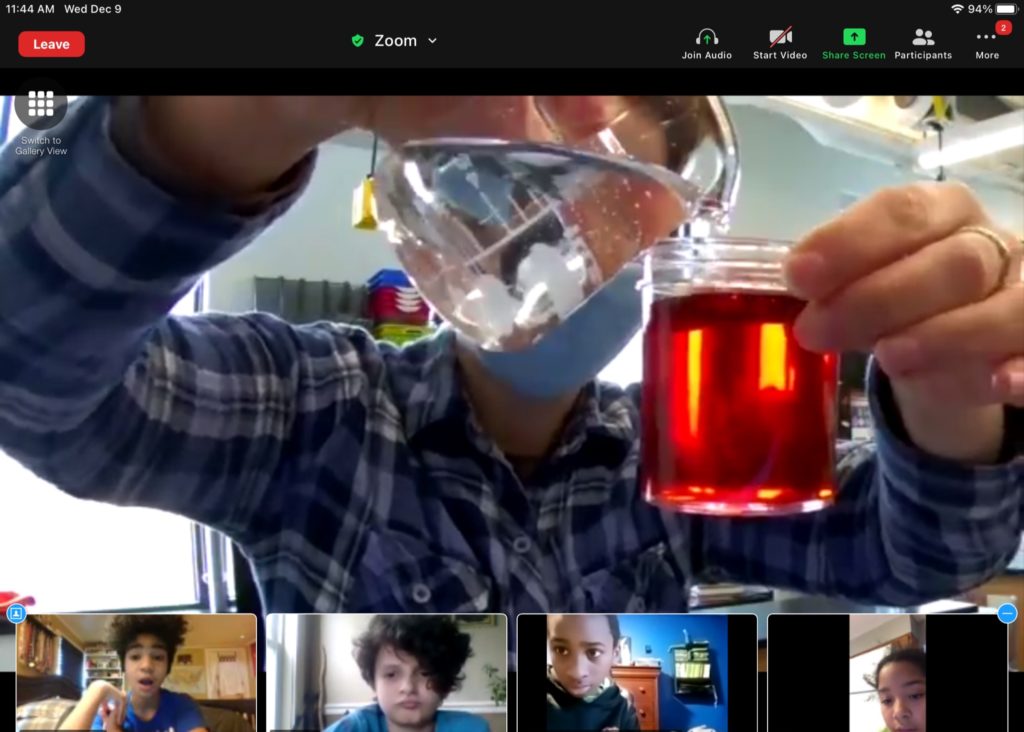
Moving on with the demonstration, Mx. Mckenzie added oil to the water. Following the same routine, students unmuted and took turns describing what they observed. The oil did create some bubbles, but it didn’t explode. The oil and water didn’t stay mixed even when they shook up the bottle again, instead separating slowly, creating a foamy yellow liquid floating on a transparent one.
After a second experiment mixing vinegar and water to make a light red liquid, Mx. Mckenzie went on, “This brings us to our question about salad dressing: if we want our salad dressing to stay mixed, why do you think the oil and water don’t mix? How do you think we can make salad dressing stop separating? Take out your kits and put your thumbs up when you have them ready, but don’t touch anything yet,” they instructed, “Do you think water or oil will be more attracted to itself?”
Then Mx. Mckenzie led a conversation about water’s properties of attraction to help students make predictions, they talked students through what drops on the pennies might look like and how the shape of the drops on the pennies– flat, rounded, or spherical– would give evidence to support the predictions that students made. A spherical shape, with strong surface tension would indicate stronger attraction to itself, like a group of people– post pandemic– all reaching their arms out to try and make a group hug.
The kits contained pennies, little cups, eye droppers, oil, water, and napkins. While Mx. McKenzie and Teaching Assistant Luis Sanchez facilitated the process, students worked through the set-up. Then the class worked all at once following the same procedures as the warm up, making predictions, counting drops of oil or water on their pennies, recording how many drops, making observations, and cleaning up to start over if their water or oil spilled. Students all sent their answers in the chat and Mx. Mckenzie recorded everyone’s answers, including their own penny which held 34 drops of water and only 22 of oil.
Based on their observations, students looked for patterns, and decided that water attracted itself more, even though oil felt stickier on their fingers.
All in all the class had a multi-faceted one hour lab designed to help students use chemistry to design their salad dressing in which students showed competence in standards related to science:
- Students made predictions based on what they learned about the forces of attraction between molecules last week, and a warm-up demonstration.
- They planned how to carry out an experiment based on the instructions and materials in their home learning kits.
- They made observations and connected it to background knowledge to explain how water and oil differ.
- They analyzed their collective results and worked together to answer the question of the day: Water with a greater number of drops on each penny, was more attracted to itself than oil.
Exposure to developmentally appropriate framing of scientific processes and concepts ensures that students remain engaged as they progress. To make this lesson developmentally appropriate the curriculum employs a simplified model of water’s polarity that will serve students well when they reach high school and it will be appropriate to learn a more complete mathematical model of chemistry.
For those who do not remember much about high school chemistry, the phenomena being explored in this lesson are adhesion and cohesion. In adhesion, water molecules cling together, creating surface tension. In cohesion, water molecules cling to other surfaces – in this case, the penny.
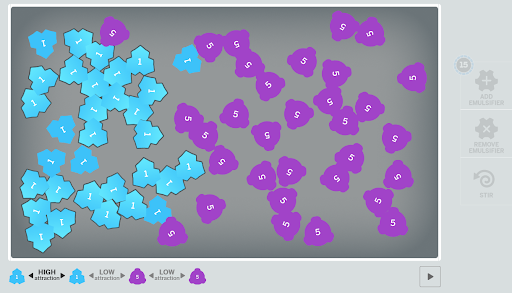
In addition to hands-on learning experiences, the new Amplify curriculum contains interactive digital models that reinforce student learning. In the simulation, the blue shapes, representing polar molecules like water have sharp angles that make it possible for them to cohere, while the purple shapes, representing non-polar molecules are more rounded, allowing students to visualize molecules like puzzle pieces.